The UK YourPhyto Scientific Study
Combining probiotic supplement and new generation phytochemical rich food capsules to aid men with indolent prostate cancer? A double blind Randomised Placebo Controlled Trial
Background:
 Emerging studies are linking poor gut health (dysbiosis) with a greater risk and progression of ca prostate (PCa). Various dietary and lifestyle factors influence dysbiosis but probiotic supplements have also been shown to improve the microbiome floral to a more favourable, less inflammatory profile. Likewise, studies have linked higher intake of phytochemical-rich foods with a lower risk of PCa and Prostatic Specific Antigen (PSA) progression. Phytochemicals have numerous direct and indirect anti-cancer properties, including reducing excess chronic inflammation and enhancing oxidative pathways, but they also act as prebiotics, which support commensal and ingested probiotic bacterial. The hypothesis for this double-blind, randomised trial is that a probiotic supplement could enhance the benefits of a phytochemical-rich supplement via this synergistic effect. A combination of phytochemical-rich food and probiotic supplements has not previously been explored in a cohort of men with PCa, hence the rationale for this study. The aims are to establish whether boosting the diet with a lactobacillus probiotic in addition to a phytochemical-rich food supplement will influence PSA progression, prostate-related symptoms and strength in men with early PCa compared to placebo.
Emerging studies are linking poor gut health (dysbiosis) with a greater risk and progression of ca prostate (PCa). Various dietary and lifestyle factors influence dysbiosis but probiotic supplements have also been shown to improve the microbiome floral to a more favourable, less inflammatory profile. Likewise, studies have linked higher intake of phytochemical-rich foods with a lower risk of PCa and Prostatic Specific Antigen (PSA) progression. Phytochemicals have numerous direct and indirect anti-cancer properties, including reducing excess chronic inflammation and enhancing oxidative pathways, but they also act as prebiotics, which support commensal and ingested probiotic bacterial. The hypothesis for this double-blind, randomised trial is that a probiotic supplement could enhance the benefits of a phytochemical-rich supplement via this synergistic effect. A combination of phytochemical-rich food and probiotic supplements has not previously been explored in a cohort of men with PCa, hence the rationale for this study. The aims are to establish whether boosting the diet with a lactobacillus probiotic in addition to a phytochemical-rich food supplement will influence PSA progression, prostate-related symptoms and strength in men with early PCa compared to placebo.
The intervention and rationale for using these nutraceutical supplements:
The advantage of using supplements, rather than diet only, in clinical studies is that healthy foods can be boosted across the day, quantified and purified. It is also more difficult to ingest sufficient probiotic on a regular basis, particularly with a UK diet. There are hundreds of commercial probiotics available to use but the rationale for using this particular probiotic complex, is that it meets all the important criteria determined by the scientific committee:
- high quantity of colony forming units (10 Billion CFU)
- built-in prebiotics (Inulin and Vitamin D) to enhance colony formation
- delayed release vegan capsule
- manufactured by a well-established UK manufacturer with high quality certification
- evaluated in previous clinical trials and found to be safe and well-tolerated.
The scientific committee decided on this particular phytochemical-rich blend following a formal review of the international scientific literature. With this data, the committee was able to build on experience of previous research from their studies and other studies from institutes across the World published over the last 10 years. The manufactures were also able to incorporate new technologies in purification, quality assurance and standardisation ensuring consistency in the candidate phytochemicals. All these factor combied has resulted in establish a new generation supplement with a number of novel and attractive features:
- combines six uniquely different food types, providing a wide spectrum of natural phytochemicals, avoiding over-consumption of one particular type
- combines both whole concentrates and extracts
- minimal levels of candidate phytochemicals are measured and standardised
- contains no foods with phytoestrogen properties
- ingredients have a high safety profile
- manufactured by a well-established UK manufacturer, with high quality certification
- evaluated in previous clinical trials and found to be safe and well-tolerated.
Methodology:
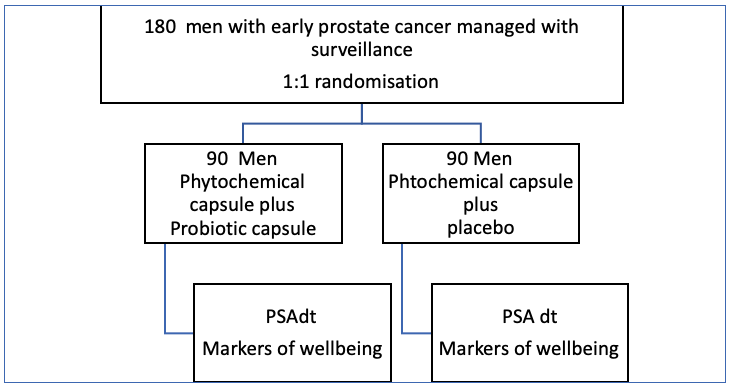
The trial cohort includes men with histologically proven, prostate canecr ( PCa ) who are not taking androgen deprivation therapy (ADT), managed with active surveillance or watchful waiting. Following written informed consent, all participating men (180) will be given the phytochemical-rich food supplement and asked to stop all other over-the-counter supplements. There will be a double-blind, randomised (1:1) allocation of the probiotic supplement or placebo. The supplements will be taken twice a day for 4 months.
Trial end points:
 Prostatic Specific Antigen doubling time (PSAdt) will be taken at baseline and 4 months together with testosterone and measures of prostate symptoms and wellbeing:
Prostatic Specific Antigen doubling time (PSAdt) will be taken at baseline and 4 months together with testosterone and measures of prostate symptoms and wellbeing:
- Vitamin D levels
- International Prostate Symptom Score (IPSS)
- International index of Erectile Function
- Grip strength as a marker of overall well being
Certification and time scales:
This national UK trial is run initially by a team from Bedford and Addenbrooke’s Cambridge University NHS trusts. The Chief Medical Officer is Mr Frazilli, a Consultant Urologist, who specialises in prostate cancer diagnosis and treatment.
The study design was peer reviewed and registered with the UK National ethics committee a (Ethics (IRAS) number: 321309) and international registered with the ISRCTN (ISRCTN 81939514). This is an independent agency were the public can view the details of the study, progress of recruitment and time scales of publications. It is affiliated with: